Implen Journal Club | June Issue |
|
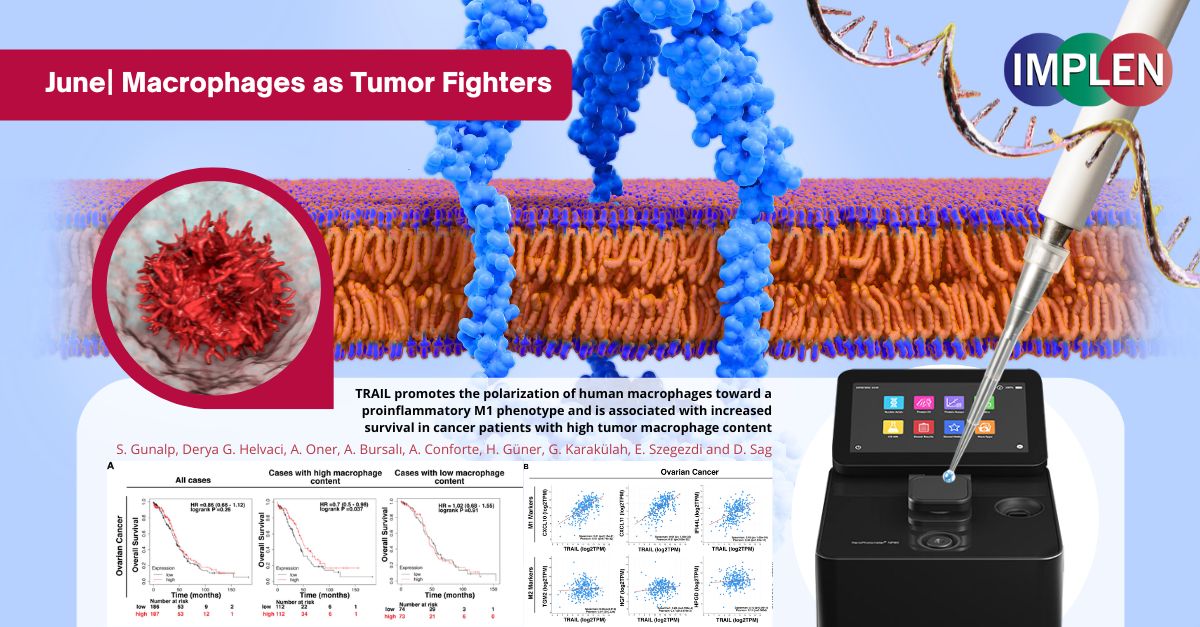
Revolutionizing Cancer Therapy: Turning Macrophages into Tumor FightersThe first issue Implen NanoPhotometer® Journal Club: Innovations in Cancer Immunotherapy Edition is highlighting the recently published study by Gunalp et. al. "TRAIL promotes the polarization of human macrophages toward a proinflammatory M1 phenotype and is associated with increased survival in cancer patients with high tumor macrophage content", which investigated how the protein TRAIL affects immune cells called macrophages. These macrophages can either fight tumors (M1 type) or support them (M2 type). The research aimed to understand how TRAIL influences this behavior. In this study, human macrophages were treated with TRAIL and other specific proteins, and then observed how they changed using several methods to measure the levels of different markers indicating whether the macrophages were of the M1 or M2 type. It also tested how well these macrophages could kill cancer cells and checked data from cancer patients to see how TRAIL levels affected survival. The results showed that TRAIL made macrophages more like the tumor-fighting M1 type and less like the tumor-supporting M2 type. When treated with TRAIL, even M2 macrophages started acting more like M1. These M1-like macrophages were better at killing cancer cells in lab tests. In patients, higher levels of TRAIL were linked to better survival rates, especially in those with many macrophages in their tumors. This study suggests that TRAIL could be a useful tool in cancer therapy by turning macrophages into more effective tumor fighters. The Implen NanoPhotometer® was used in this study to check the purity of RNA samples. This step was crucial to ensure that the RNA samples were of high quality and free from contaminants, which is essential for accurate downstream analyses such as RNA sequencing and quantitative PCR (qPCR). |
|
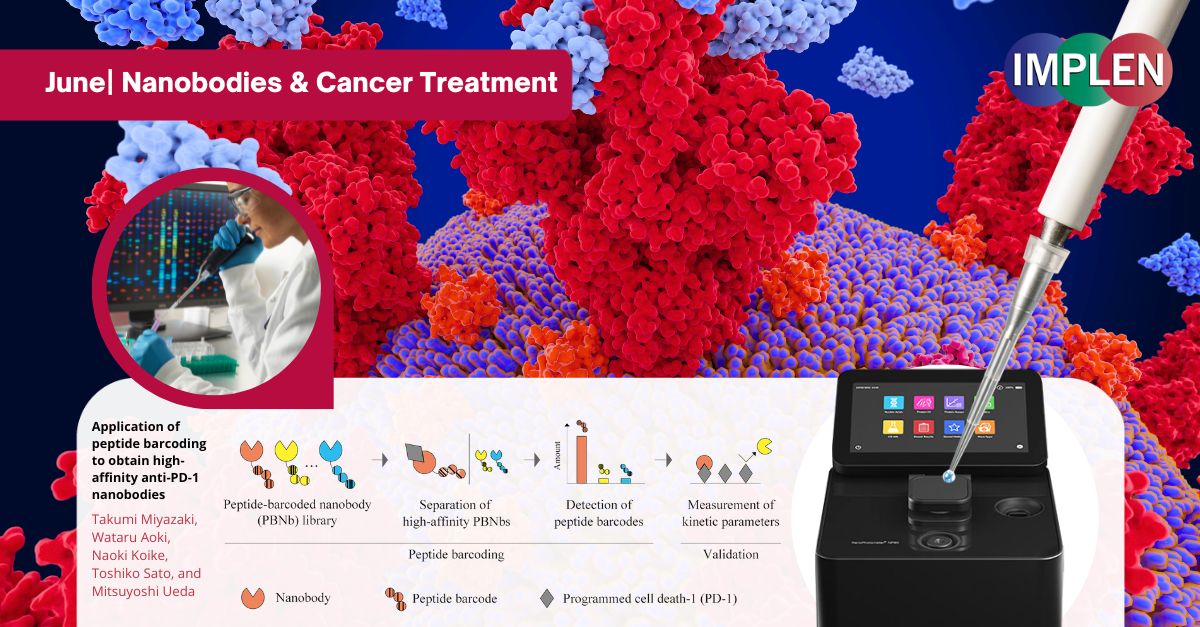
Application of peptide barcoding to obtain high-affinity anti-PD-1 nanobodiesNext, the Journal Club is featuring the groundbreaking study, "Application of peptide barcoding to obtain high-affinity anti-PD-1 nanobodies," by Miyazaki et al. This research explored the development of nanobodies, a promising alternative to conventional antibodies in cancer treatment. Derived from camels, nanobodies were smaller and more stable, making them ideal for targeting cancer cells. This study aimed to streamline the discovery of these nanobodies using peptide barcoding, a technique that tagged each nanobody with a unique barcode for efficient screening. By creating a library of thousands of nanobodies, the researchers identified three that were particularly effective in blocking cancer cell interactions. These nanobodies showed significant potential in inhibiting the PD-1/PD-L1 interaction, a crucial target in cancer therapy. This research highlighted the utility of peptide barcoding in rapidly and reliably identifying high-affinity nanobodies, offering promising tools for cancer immunotherapy. Results from this study suggest that these nanobodies could surpass traditional antibodies, leading to more effective cancer treatments. The NanoPhotometer® was used in this study to measure the concentrations of DNA fragments. This step ensured that the correct amounts of DNA were used in further processes such as sequencing and transformation, which were critical for the accurate identification and quantification of high-affinity nanobodies. |
|
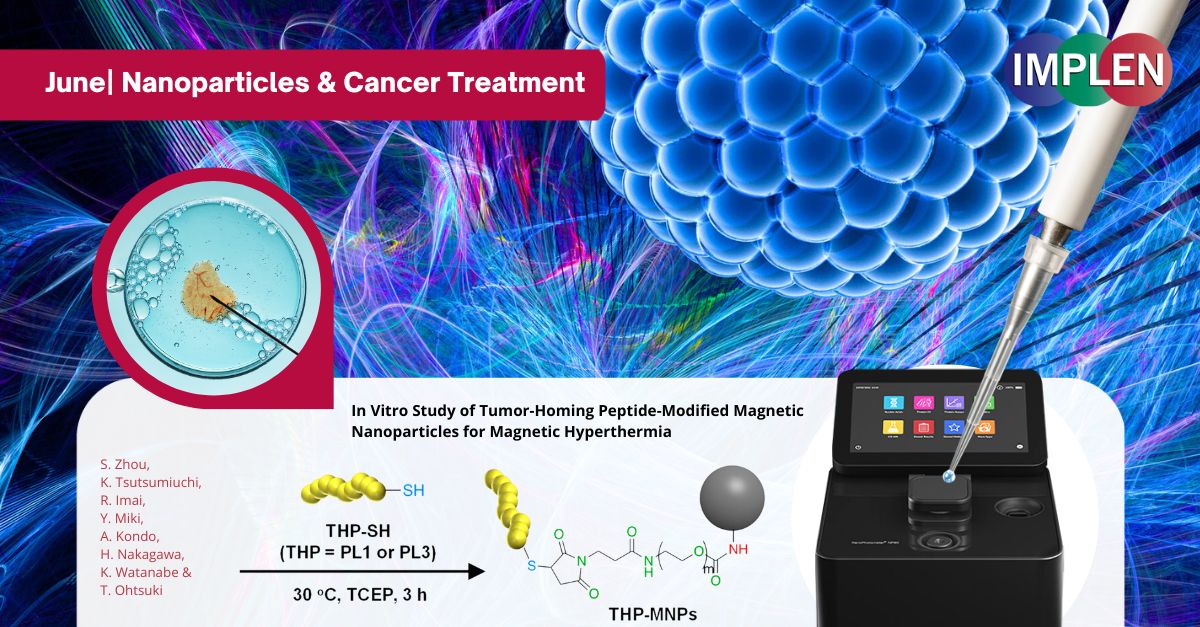
Breakthrough in Cancer Immunotherapy: Precision Targeting with Magnetic NanoparticlesOur next issue highlights the recent study by Zhou et al., "In Vitro Study of Tumor-Homing Peptide-Modified Magnetic Nanoparticles for Magnetic Hyperthermia," which explored a novel cancer treatment method using heat to target tumors. The researchers modified magnetic nanoparticles (MNPs) with tumor-homing peptides (THPs) to enhance their effectiveness in targeting cancer cells, offering a promising advancement in cancer immunotherapy. This study focused on two specific peptides, PL1 and PL3, which were attached to the MNPs. These peptides significantly improved the nanoparticles' ability to adhere to and penetrate cancer cells compared to unmodified nanoparticles. When exposed to an alternating magnetic field (AMF), these modified nanoparticles generated heat that effectively killed the cancer cells, with PL3-MNPs showing particularly high efficacy. Remarkably, even without the magnetic field, PL3-MNPs induced cancer cell death through ferroptosis, a process where iron from the nanoparticles causes oxidative damage to cell membranes. The findings are promising, demonstrating that THP-modified MNPs, especially those with PL3, significantly enhance the precision and effectiveness of targeting and destroying cancer cells while minimizing harm to healthy tissues. This innovative approach could lead to safer and more effective cancer treatments, potentially complementing existing immunotherapy methods. The study suggests further testing in living organisms to validate these results and explore potential clinical applications. The NanoPhotometer® was used in this study to determine the MNP weight concentrations (without the weight of THP moiety) of MNPs and THP-modified MNPs by measuring the absorbance of the MNPs at 400 nm. |
|
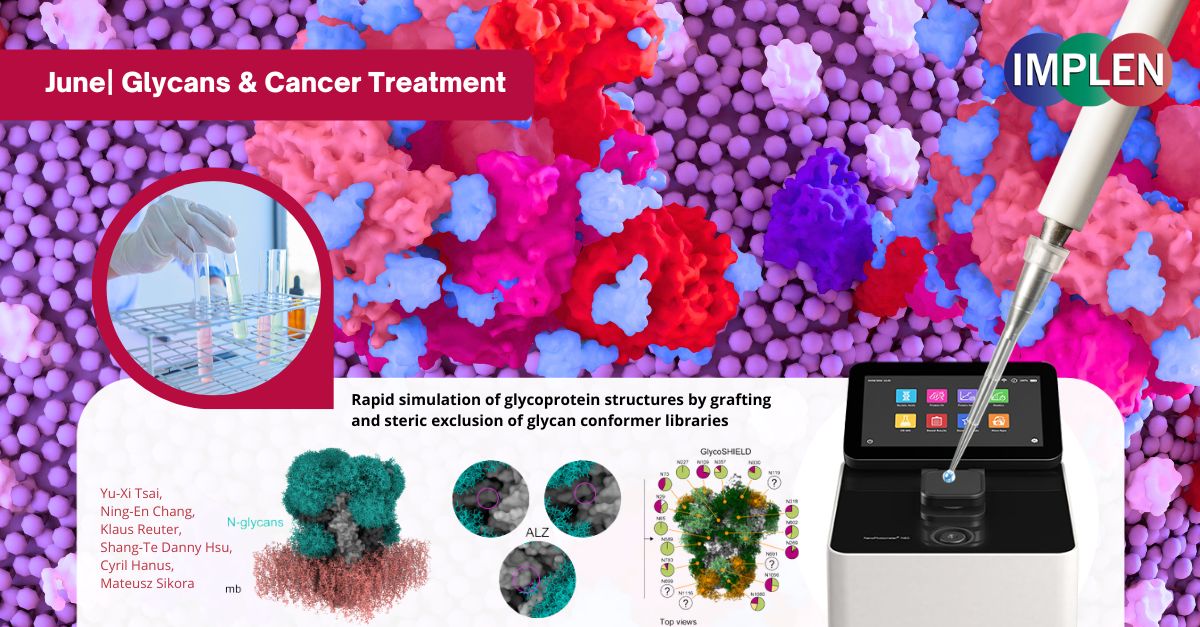
Revolutionizing Cancer Immunotherapy: Introducing GlycoSHIELD – A Breakthrough Tool for Faster, Easier Glycan ModelingOur last issue is featuring a recent study by Tsai et al. that introduced GlycoSHIELD, a new tool for predicting how glycans (complex sugars attached to proteins) affect protein structure and function. Previously, modeling these complex structures required significant computing power and time, but GlycoSHIELD simplifies and accelerates the process. GlycoSHIELD simulates the appearance and behavior of glycans by attaching precomputed models to protein structures. Tested for accuracy through various experimental methods, it was demonstrated to provide detailed insights into how these glycan "shields" cover and protect proteins on cell surfaces. This has major implications for cancer immunotherapy, as cancer cells often have unique glycan shields that help them evade the immune system. GlycoSHIELD can model these shields, enabling researchers to understand how they protect cancer cells and how therapeutic antibodies or immune cells might interact with them. This deeper understanding could lead to the design of more effective treatments that bypass or modify these glycan shields, improving immune detection and targeting of cancer cells. The Implen NanoPhotometer® N60 UV-Vis spectrophotometer was used in this study to determine the protein concentrations using the UV absorbance at 280 nm. |
©2024 Implen. All rights reserved.